21-Years of Botamedi
What we have done
40
Patents
100
Studies
100
Products
Our Mission
We provide marine phytochemical-based therapeutics
Botamedi Inc. aims to establish a new-paradigm therapeutic approach based on both safe and effective marine phytochemicals, in order to prevent and manage the currently-incurable chronic degenerative disease that have prevailed in the 21st century.
2001-2022
21-Years of Botamedi
40 Patents
- 30 Registered and 10 pending patents in 7 major areas of health science.
- 75+ Trade Marks in Korea, China, Japan and USA.
100+ Studies
- 20+ Chinical trials including Phase I and II studies.
- 80+ Cell and animal toxicity and efficacy studies.
FDA Approvals
- NDI*(SEAPOLYNOL®-Ecklonia cava extract) by the US FDA in 2008.
- NFI* (SEAPOLYNOL®-Ecklonia cava phlorotannins) by the EU in 2018.
- Health-Functional Ingredient Approval by the KFDA (MFDS) in 2012.
- IND* (PH100) by the US FDA in 2013.
100+ Products
- 50+ Dietary Supplements.
- 40+ Cosmetics and Functional Creams.
- 10+ Berverages and SEANOL® Foods.
Novel Materials
Novel synthetic compounds derived from marine phytochemicals have been developed for potential applications in therapeutics and nanomaterial science
- *NDI: New Dietary Ingredient authorized by the US FDA after a strict review of safety and standardization.
- *NFI: The European version of NDI, with 2-3 times the difficulty
- *IND: Investigational New Drug approved by the US FDA as a new clinical trial drug after a deep and thorough review of GMP, toxicology, and efficacy studies.
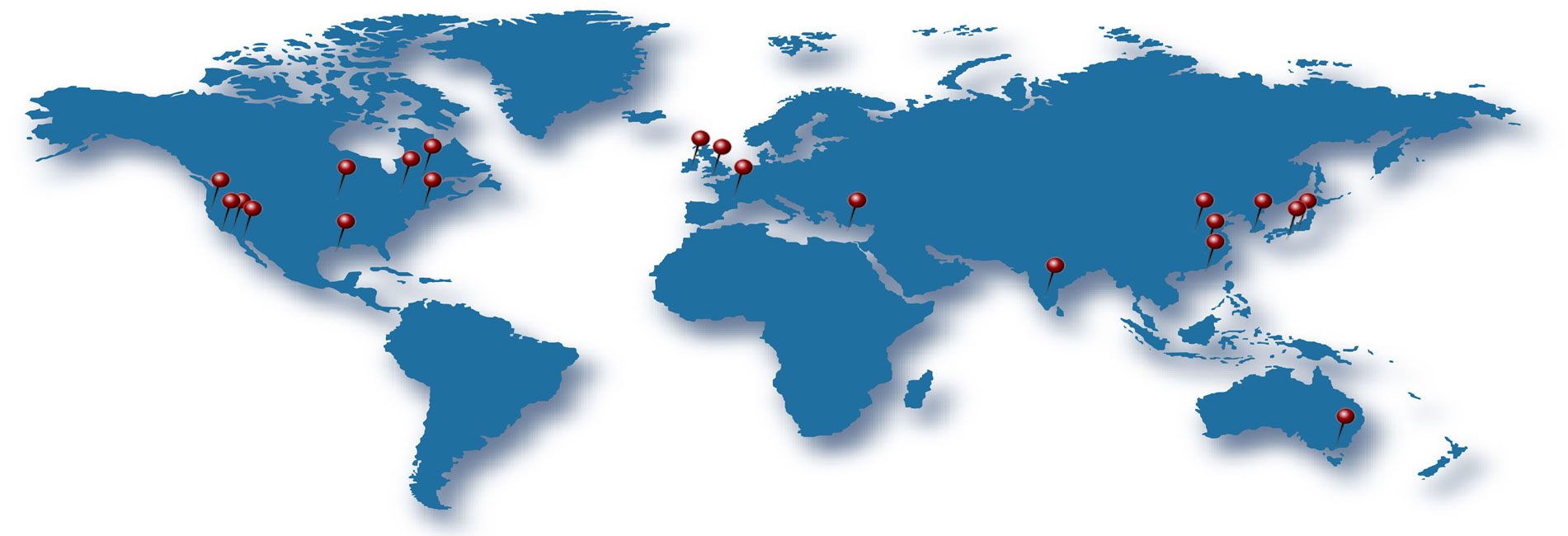
Our Collaborations
World-wide Research Collaborations on Marine Phytochemicals
Since marine phytochemicals have hardly been studied in the past, so many new scientific revelations have been made by specialists in every field of chemistry and the life sciences. Botamedi has been working together with a number of research organizations and scientists around the world to accomplish this enormous task of “from under the sea to enabling therapeutics”.